1FIN - chain A | Cyclin-dependent kinase 2
Structure information
| PDB: | 1FIN |
| PubMed: | 7630397 |
| Release date: | 1997-01-27 |
| Resolution: | 2.3 Å |
| Kinase: | CDK2 |
| Family: | CDK |
| Group: | CMGC |
| Species: | HUMAN |
| Quality Score: | 8 |
| Missing Residues: | 0 |
| Missing Atoms: | 0 |
| DFG conformation: | in |
| αC-helix conformation: | in |
| Salt bridge KIII.17 and EαC.24: | Yes (3.1Å) |
| ASP rotation (xDFG.81) : | 355° |
| PHE rotation (xDFG.82) : | 16° |
| Activation loop position: | -3.3Å |
| αC-helix position: | 17.4Å |
| G-rich loop angle: | 58.6° |
| G-rich loop distance: | 17.5Å |
| G-rich loop rotation: | 61.5° |
Other models from this PDB:
2D & 3D views
Binding pocket waters
The following waters were found in the defined clusters:
I3
H-bond ligand
I5
H-bond ligand
I6
H-bond ligand
Binding pocket sequence
| Uniprot | EKIGEGTYGVVYKVALKKITAIREISLLKELNPNIVKLLDVYLVFEFLH-QDLKKFMDAFCHSHRVLHRDLKPQNLLILADFGLA |
| Structure: | EKIGEGTYGVVYKVALKKITAIREISLLKELNPNIVKLLDVYLVFEFLH_QDLKKFMDAFCHSHRVLHRDLKPQNLLILADFGLA |
Modified residues
No modified residues identified.
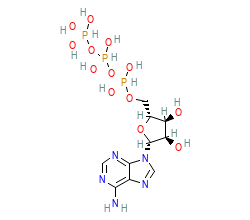
Orthosteric ligand
- Download image
- LABELS
- KLIFS residue #
- Amino Acid
- None
- COLORS
- Interaction types
- KLIFS (all res.)
- KLIFS (interacting res.)
- None
- OTHER
- Show/hide non-interacting res.
- En/disable resizing interacting res.
This ligand targets the following (sub)pockets:
| Main pockets | |
|---|---|
| Front | |
| Gate | |
| Back | |
| Subpockets | |
|---|---|
| FP-I | |
| FP-II | |
| BP-I-A | |
| BP-I-B | |
| BP-II-in | |
| BP-II-A-in | |
| BP-II-B-in | |
| BP-II-out | |
| BP-II-B | |
| BP-III | |
| BP-IV | |
| BP-V | |
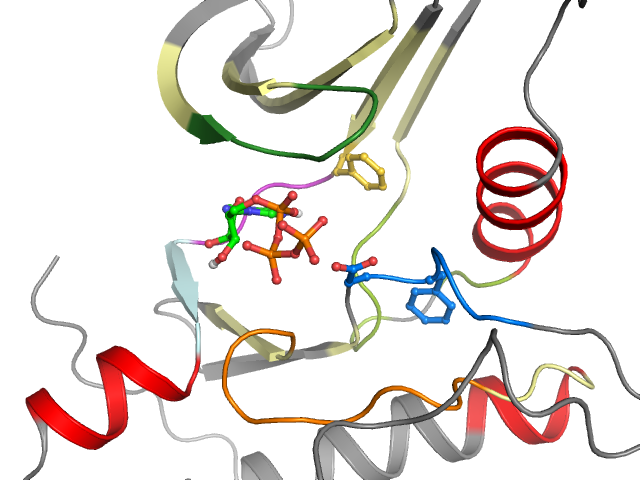
Kinase-ligand interactions
■ Hydrophobic ♦ Aromatic face-to-face ♦ Aromatic face-to-edge ▲ H-bond donor ▲ H-bond acceptor ● Ionic positive ● Ionic negative
| I | g.l | II | III | αC | |||||||||||||||
| 1 E 8 | 2 K 9 | 3 I 10 | 4 G 11 | 5 E 12 | 6 G 13 | 7 T 14 | 8 Y 15 | 9 G 16 | 10 V 17 | 11 V 18 | 12 Y 19 | 13 K 20 | 14 V 30 | 15 A 31 | 16 L 32 | 17 K 33 | 18 K 34 | 19 I 35 | 20 T 47 |
| ■ | ■ | ■ | ■ | ||||||||||||||||
| αC | b.l | IV | |||||||||||||||||
| 21 A 48 | 22 I 49 | 23 R 50 | 24 E 51 | 25 I 52 | 26 S 53 | 27 L 54 | 28 L 55 | 29 K 56 | 30 E 57 | 31 L 58 | 32 N 59 | 33 P 61 | 34 N 62 | 35 I 63 | 36 V 64 | 37 K 65 | 38 L 66 | 39 L 67 | 40 D 68 |
| IV | V | GK | hinge | linker | αD | αE | |||||||||||||
| 41 V 69 | 42 Y 77 | 43 L 78 | 44 V 79 | 45 F 80 | 46 E 81 | 47 F 82 | 48 L 83 | 49 H 84 | 50 _ _ | 51 Q 85 | 52 D 86 | 53 L 87 | 54 K 88 | 55 K 89 | 56 F 90 | 57 M 91 | 58 D 92 | 59 A 93 | 60 F 117 |
| ▲ | ■♦ | ■▲ | |||||||||||||||||
| αE | VI | c.l | VII | VIII | x | ||||||||||||||
| 61 C 118 | 62 H 119 | 63 S 120 | 64 H 121 | 65 R 122 | 66 V 123 | 67 L 124 | 68 H 125 | 69 R 126 | 70 D 127 | 71 L 128 | 72 K 129 | 73 P 130 | 74 Q 131 | 75 N 132 | 76 L 133 | 77 L 134 | 78 I 135 | 79 L 143 | 80 A 144 |
| ■ | |||||||||||||||||||
| DFG | a.l | ||||||||||||||||||
| 81 D 145 | 82 F 146 | 83 G 147 | 84 L 148 | 85 A 149 | |||||||||||||||
Binding affinities
ChEMBL ID:CHEMBL14249Bioaffinities: 8 records for 3 kinase(s)
| Species | Kinase (ChEMBL naming) | Median | Min | Max | Type | Records |
|---|---|---|---|---|---|---|
| Homo sapiens | MAP kinase-activated protein kinase 2 | 5.2 | 4.9 | 5.6 | pIC50 | 3 |
| Homo sapiens | Mitogen-activated protein kinase kinase kinase 7 | 5.6 | 5.6 | 5.6 | pKd | 1 |
| Homo sapiens | Tyrosine-protein kinase JAK2 | 5.9 | 5.9 | 5.9 | pKd | 4 |